|
GR9677 #59
|
|
|
|
|
Alternate Solutions |
insertphyspun
2011-05-26 13:46:20 | Of course, the E=Pt approach is probably best, but I was going too fast to see that P=10kW. My alternate solution uses the uncertainty principle:
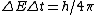
where 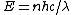 . Thus, . Thus,
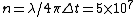
Close enough, and no need to convert Watts to eV/s. |  | proctort
2009-09-10 20:00:28 | First time posting, defaults to NEC apparently. This one is properly labeled:
Given that this answer is virtually illegible, I'm writing out my own answer:
The energy of a beam of light is  , where , where  is the number of photons and is the number of photons and 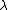 is the wavelength. As power is defined as P=\frac{E}{t}, where is the wavelength. As power is defined as P=\frac{E}{t}, where  is the time over which energy is given off, we have is the time over which energy is given off, we have 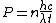 , which, solving for , which, solving for  gives n = \frac{P \lambda t}{h c}. gives n = \frac{P \lambda t}{h c}.
In SI units, 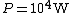 , , 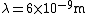 ..., which gives ..., which gives
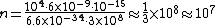 |  |
|
|
Comments |
digitalhikes
2019-07-08 11:04:17 | Happy to go to your post, i\\\\\\\'m by all accounts forward to more and more dependable articles and that i figure we have a tendency to as a full would like to convey such Brobdingnagian numbers of excellent articles, post like Hostpapa Promo Code 2019 to important to us. \\\\r\\\\n |  | insertphyspun
2011-05-26 13:46:20 | Of course, the E=Pt approach is probably best, but I was going too fast to see that P=10kW. My alternate solution uses the uncertainty principle:
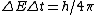
where 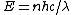 . Thus, . Thus,
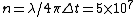
Close enough, and no need to convert Watts to eV/s.
Setareh
2011-10-07 09:52:25 |
I think in this case you have forgotten "c" in denominator of n=lambda/4*pi*t. rnActually the real equation for n is:rnn=lambda/4*pi*c*trnif you put c= 3*10^8 , you will see that it is larger than the correct answer.rnI think you have to write: n<=lambda/4*pi*c*trnyou have only found the boundary, but the power will determine which n is eligible for this problem.
|
|  | proctort
2009-09-10 20:00:28 | First time posting, defaults to NEC apparently. This one is properly labeled:
Given that this answer is virtually illegible, I'm writing out my own answer:
The energy of a beam of light is  , where , where  is the number of photons and is the number of photons and 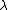 is the wavelength. As power is defined as P=\frac{E}{t}, where is the wavelength. As power is defined as P=\frac{E}{t}, where  is the time over which energy is given off, we have is the time over which energy is given off, we have 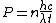 , which, solving for , which, solving for  gives n = \frac{P \lambda t}{h c}. gives n = \frac{P \lambda t}{h c}.
In SI units, 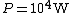 , , 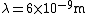 ..., which gives ..., which gives
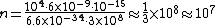
alisonsparkles
2012-10-02 01:57:42 |
Doesn't the wavelength = 600*10^-9? This gives me an answer of 1/3*10^10...
|
calcuttj
2014-09-20 07:21:37 |
Technically the wavelength is 6E-7 or 600E-9
|
|  | proctort
2009-09-10 19:53:33 | Given that this answer is virtually illegible, I'm writing out my own answer:
The energy of a beam of light is 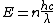 , where , where  is the number of photons and is the number of photons and 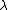 is the wavelength. As power is defined as is the wavelength. As power is defined as 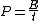 , where , where  is the time over which energy is given off, we have is the time over which energy is given off, we have 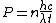 , which, solving for , which, solving for  gives gives 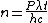 . .
In SI units, 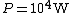 , , 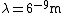 ..., which gives ..., which gives
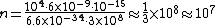 |  | isina
2008-10-18 13:17:55 | where does that 1.602E-19 come from?
akbar5223
2008-10-28 08:12:41 |
The laser power is given in W=J/s, so it is necessary to convert the power from J/s to eV/s, where 1 eV = 1.602E-19 J.
|
naroays
2008-11-03 02:41:15 |
Why should we convert to eV/s?
The SI unit of energy is Joules, and 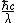 is in Joules, as is Power*time, because it's Joules/second * second = Joules is in Joules, as is Power*time, because it's Joules/second * second = Joules
|
naroays
2008-11-03 02:51:08 |
Ah, I just noticed the soln in the website doesn't use SI units. Nevermind
|
|  |
|
| Post A Comment! |
You are replying to:
First time posting, defaults to NEC apparently. This one is properly labeled:
Given that this answer is virtually illegible, I'm writing out my own answer:
The energy of a beam of light is  , where  is the number of photons and 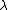 is the wavelength. As power is defined as P=\frac{E}{t}, where  is the time over which energy is given off, we have 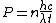 , which, solving for  gives n = \frac{P \lambda t}{h c}.
In SI units, 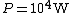 , 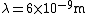 ..., which gives
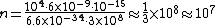 |
Bare Basic LaTeX Rosetta Stone
|
LaTeX syntax supported through dollar sign wrappers $, ex., $\alpha^2_0$ produces  . .
|
| type this... |
to get... |
| $\int_0^\infty$ |
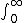 |
| $\partial$ |
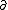 |
| $\Rightarrow$ |
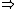 |
| $\ddot{x},\dot{x}$ |
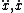 |
| $\sqrt{z}$ |
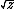 |
| $\langle my \rangle$ |
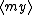 |
| $\left( abacadabra \right)_{me}$ |
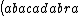_{me}) |
| $\vec{E}$ |
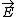 |
| $\frac{a}{b}$ |
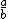 |
|
|
|
|
The Sidebar Chatbox...
Scroll to see it, or resize your browser to ignore it... |
|
|
|
